Top Emerging Careers in the Pharmaceutical Industry that Didn’t Exist 10 Years Ago
The pharmaceutical industry is undergoing an irreversible transformation, where there is a fundamental shift in drug development, patient care, and manufacturing. A decade ago, careers in the pharmaceutical industry were dominated by traditional roles, such as Research Scientist, Clinical Research Associate, and standard biostatistician. While these roles remain as important as they are, a new, highly specialized Hybrid career has emerged, born from biology, big data, and technology.
These roles didn’t exist 10 years ago, as the foundational technologies like cloud computing, machine learning, and wearable devices did not exist; this also resulted in regulatory acceptance of real-world data. The shift from a volume-based, generalised care model to a value-based personalised medicine approach has created a demand for pharma professionals.
This article will help you understand the careers in the Pharmaceutical Industry that are available now, detailing industry demands, future scope, and the pathways for students.
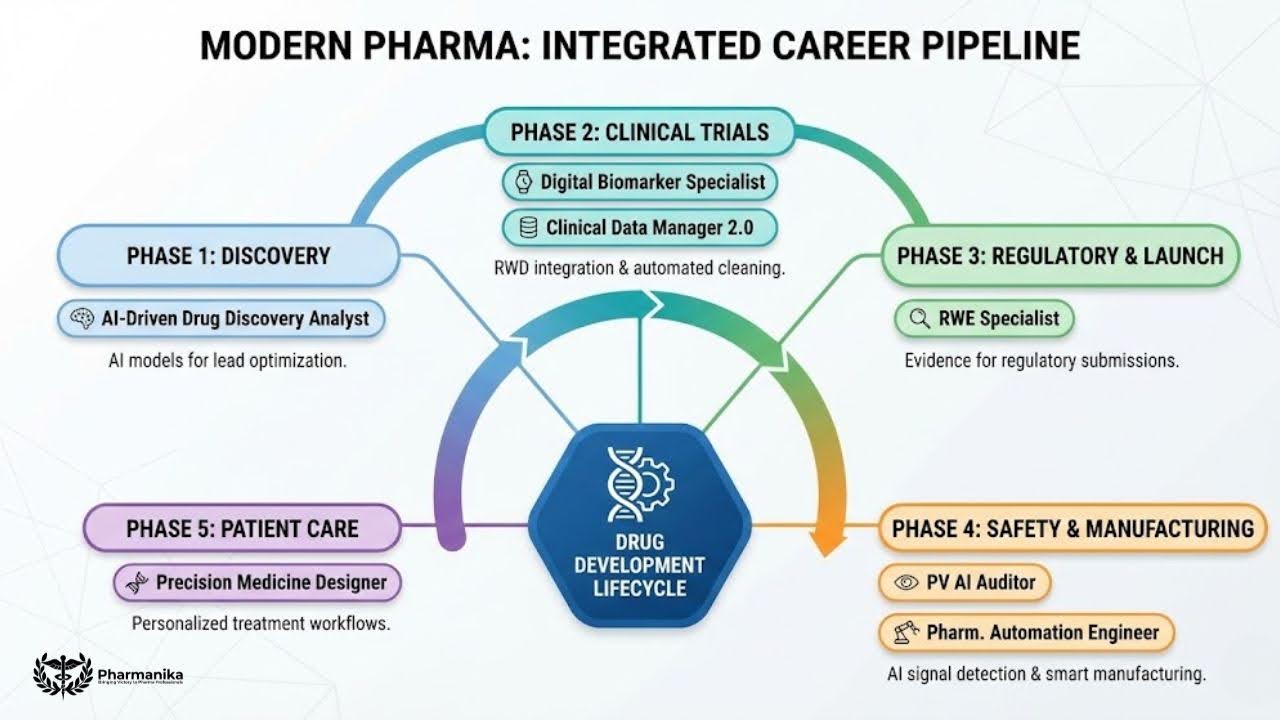
The New Emerging Careers in the Pharmaceutical Industry
1. Digital Biomarker Specialist
The specialist defines, validates, and integrates data from consumer-grade and medical-grade digital health technologies like smart watches, continuous glucose monitors, or specialised smartphone apps for the clinical trials. They measure the movement, sleep quality, voice patterns data and turn them into quantifiable measures for the disease progression or treatment efficacy.
Why this role didn’t exist before:
The adoption of FDA/EMA acceptable wearable technology and regulatory guidelines to support RWD from these sources is a recent development; therefore, these data were considered not clinical a decade ago.
Industry Demands
The need for objective, continuous patient monitoring to lower clinical trial costs, increase patient diversity, and move away from site-based measurements.
Future scope
Essential in decentralised and virtual trials, particularly in neurology, psychiatry, and chronic disease management.
Skills& Certifications
- Python/R for time series data analysis
- Signal Processing,
- Biostatistics and a deep understanding of sensor validation protocols like ISO Standards.
- There are online courses available in Health Informatics or Clinical Trial Design for Wearables.
Career growth
In mid-career, salaries are often competitive for biostatisticians, in the 5 to 6-figure range, which differs based on the specialised CROs.
2. Clinical Data Manager 2.0 (AI-Augmented)
This is an evolution of the traditional Clinical Data manager role, which involves manual Data Clarification Form generation and Query Resolution. This 2.0 job involves overseeing AI Text, machine learning systems that perform predictive data cleaning, automotive query management, and identify complex data patterns like site fraud or protocol deviations in real time.
Why didn’t this Job Exist Before?
The use of predictive AI text to clean and harmonize datasets was not feasible. Manual Data review and basic range checks are the only sources.
Industry Demands
The multi-source data, like EHRs, Wearables, and labs, demand automated cleaning and harmonization. The AI text in the pharma industry is directly involved in this role that helps to shift the human task from execution to supervision and strategic validation.
Future Scope
This Clinical Data Manager role will become the data Quality architect who focuses on CDISC standardisation and managing the data systems across global trials.
Skills & Certifications
- Learn Electronic Data Capture systems like Medidata Rave,
- Fluency in SQL/PYTHON for query,
- Specialised training in AI Text tools for data governance and detection.
- Certifications like Certified Clinical Data Manager will give a strong foundation to this role.
Career Growth
The Clinical Data Manager role offers rapid progression into clinical data science or head of data governance, which exceeds traditional CDM salary benchmarks by 15-20%
3. Real-World Evidence Specialist
This specialist is a scientific and business translator who studies using real-world data like electronic health records, insurance claims, and patient registries to generate evidence for regulatory submissions, payer negotiations, and post-market safety monitoring.
Why it Didn’t Exist Before
Regulatory bodies like the FDA were doubtful about accepting the RWE to make the core regulatory decisions. The tools to link, de-identify, and analyse did not exist.
Industry Demands
The pressure from the payers and governments to demonstrate value and comparative effectiveness outside the controlled trials.
Future scope
This role offers the development of synthetic control arms that reduce the need for traditional phase 3 placebo groups.
Skills & Certifications
- Epidemiology, health economics, and outcomes research (HEOR)
- statistical programming (SAS/R/Python)
- Deep understanding of data linkage and privacy laws
- A Master’s in Health Economics OR Public Health will give a good entry point.
4. AI-Driven Drug Discovery Analyst (Role Powered by AI in the Pharma Industry)
A computation biologist who designs, trains, and deploys the machine learning models to analyse genomic data, chemical libraries, and predict the toxicity through in silico models. This role represents the importance of AI in the pharma industry, where algorithms accelerate how new medicines are discovered and developed.
Why didn’t it Exist Before?
This role emerged only recently because AI in the pharma industry required access to High-performance computing and was not readily accessible and vast; the datasets required training and effective deep learning models, like Millions of protein structures and chemical properties, which did not exist in their current form.
Industry Demands
The cost and failure rate of traditional drug discovery will be decreased as this role offers an accelerated and promising discovery of drugs.
Future Scopes
The lead analyst in automated systems and robotics labs. This role will be the future of careers in pharmaceutical R&D.
Skills & Certifications to Build a Career in AI in the Pharma Industry
- Learn frameworks like TensorFlow/PyTorch
- Chemoinformatics, bioinformatics, and strong molecular biology knowledge
- Focus on the online course in AI in Drug Discovery
5. Pharmaceutical Automation Engineer
Designing and maintaining robotic, cloud-controlled, and highly flexible manufacturing and lab automation systems, and the responsibilities involved in this role. They make sure that the integrity of the data generated by an automated process is maintained.
Why it Didn’t Exist Before
The move to fully digital, flexible manufacturing systems that can switch production with software-made protocols is a recent development.
Industry Demands
There is demand in manufacturing to support personalised medicines and global supply chains.
Future Scope
Implementing digital Twins of the manufacturing plants and AI in the pharma industry to predict and prevent batch failures.
Skills& Certifications
Learn Internet of Things, SCADA, Cloud platforms like AWS, Azure, as applied to GxP environments
6. Pharmacovigilance AI Auditor:
This role involves auditing the Drug safety by performance and ML models used for high-volume case intake, signal detection, and adverse event reporting from the unstructured sources like social media and patient forums, etc.
Why didn’t it Exist Before?
The volume of unstructured data is too much for traditional systems, and the necessity to use AI for signal detection is new.
Industry Demand
Growth in adverse event report forms from the digital sources, which is combined with the global regulatory mandates for safety signal detection.
Future Scope
Designing ethical and fair AI systems to avoid algorithmic bias towards the patient populations.
Skills& Certifications
- Pharmacovigilance, like MedDRA/ICH
- Experience with AI tools
- Signal detection
- Advanced Diploma in AI Integration in Drug Safety and Compliance
7. Precision Medicine Workflow Designer
This role focuses on the data flow design required to deliver a personalised treatment. They create an end-to-end pathway from the patient’s genetic test, through the analysis of bioinformatics, to the prescription of a dose that is tailored to the patient’s pharmacogenomic profile.
Why didn’t it Exist Before?
Targeted therapies didn’t exist before, and the cost of genome sequencing was higher; the regulatory framework is still forming for these diagnostics.
Industry Demands
The shift from the highly targeted oncology and rare disease treatments requires robust and error-free logistics that move generic data along with the pills.
Future Scope
Real-time genetic sequencing into hospital AI systems for immediate therapeutics.
Skills& Certifications
- Bioinformatics, clinical workflow designing
- Understanding of companion diagnostics and regulatory requirements
Conclusion:
The top emerging careers in the pharmaceutical Industry are hybrid roles that demand expertise in computational tools for the future.
The path to the high-value roles requires continuous learning, with a focus on the AI tools and regulatory frameworks, and digital health.
The pharma professionals of the next decade will be those who ensure that the technology that is developed is safer, faster, and more personalised treatments for patients globally.




