HOW TO START A CAREER IN CLINICAL DATA MANAGEMENT AFTER PHARMACY (COMPLETE STUDENT GUIDE)
If you are a pharmacy graduate looking for a career that has good growth and stability and also opens the doors to global opportunities, then Clinical Data Management (CDM), is the one of the best options for you. CDM is a mixture of pharma knowledge and data skills which makes it an ideal for students who want to start their career in technical rather than just getting into sales and marketing. This article helps you to understand how CDM works, CDM Jobs & its salaries, skills, CDM Tools, SAS usage in CDM, how you can start your journey as a fresher. Let’s dive in.
What is Clinical Data Management?
In every clinical trial huge amounts of data is generated, like patient details, their symptoms, lab results, side effects, their response to the treatment, and their medical history etc.
Clinical Data Management is a process of collecting, validating, cleaning, organizing and preparing the data that is generated during the clinical trails for analysis and regulatory submissions.
CDM mainly focuses on the following:
- Data Accuracy
- Data Quality
- Data Consistency
- Regulatory compliance
- Preparing final data for analysis
Clinical Data Management makes sure that every data produced during the clinical trials is accurate and trustable so that companies can make safe decisions and get approval.
Why Pharmacy Students are Perfect Fit For CDM Jobs
Students in the Pharmacy background already have a good understanding about the drugs, diseases, clinical trials, and some other medical terminologies that can give rise to a huge advantage in the CDM field. Here are the reasons why CDM is popular among the Pharmacy Graduates whether it be B Pharmacy or Pharm D.
- A Strong demand from the CROs and Pharma Companies
- Its technical Job that also involves analytics
- It has fast career growth where you can almost become a senior within 3-5 years
- Provides opportunities to work with global clients
- It has a high job stability and it has remote/hybrid flexibility also
- Skills such as SAS Programming, risk based monitoring, medical coding, biometrics etc can provide a pathway to a specialised career.
CDM professionals exceptionally have many areas of expertise to work with such as clinical Research Organisations, Pharmaceutical and biotech companies, Hospitals that conduct Clinical Trails, IT companies that offer Pharma Data Management Services and Global Consulting Firms, Data analytic Companies.
Top Recruiters For CDM in India:
IQVIA, ICON, Paraxel, Syneos Health, Convance, Accenture, Cognizant, Novartis, Pfizer, GSK, ArisGlobal, Freyr, Indegene.
Role In Clinical Data Management Career:
The following are the Key CDM job profiles that you can target to achieve a career.
Entry-Level Roles ( Perfect for B Pharm/M Pharm Freshers)
- Clinical Data Coordinator
- Clinical Data Associate
- CDM Trainee
- Data Validation Executive
- Medical Coder
- Lab Data Reviewer
Mid-Level Roles ( 2-4 Years Experience)
- Data Analyst
- Data ValidatiON Specialist
- Query Management Specialist
- SAE Reconciliation Specialist
- Data Programmer
Advanced Roles (5+ Years Experience)
- Lead Clinical Data Manager
- Principal Data Manager
- CDM Project Manager
- CDM Quality Analyst
- Clinical Database Architect
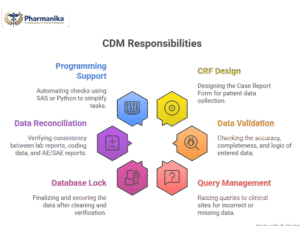
Key Responsibilities In CDM
- Case Report Form (CRF) Design
The Case Report Form is needed for collecting the patient data during the Clinical Trails, and therefore the CDM Professional will generate a design for the form.
- Data Entry & Data Validation
The CDM professional should check if the data entered from the patients and the sites is correct, complete and logical.
- Query Management
If the data entered has any incorrect data such as wrong age, missing lab value, the CDM professional will raise a query to the clinical site.
- Database Lock
The CDM professional makes sure that the data entered is clean and finalise it before the analysis.
- Data Reconciliation
To check if the lab reports, medical coding data and AE/SAE reports are matching or not.
- Programming Support
Skills like SAS or Python will help the CDM professionals to automate the check that helps to make their job easy.
Essential skills Required For the Clinical Data Management Career
As a fresher, if you learn these skills, this might help you to achieve a good career in the CDM Career.
Technical Skills
- Understanding Clinical Trials (phase 1 -4)
- Knowledge of ICH-GCP guidelines
- Basics of CRF design
- Query Management
- Data validation rules
- Exposure to CDM tools
- Basics Of SAS in clinical Research
- Understanding of Medical Terminology and drug classifications
Soft Skills
- Communication Skills
- Attention to Details
- Analytical Thinking
- Time management
- Logical Decision Making
Important CDM tools You should Learn (Beginner+ Advanced)
Here are some of CDM Tools that you should at least have a basic understanding if you are a fresher and want to stand out from the other applications.
Commonly Used Tools
- Medidata Rave
- Oracle Clinical
- Veeva Vault CDMS
- FortressIQ
- Omnicomm TrailMaster
- ClinTrail
Medical Coding Tools
- MedRA
- WHO-DDE
SAS in Clinical Research & CDM
SAS is one of the most widely used and important tools in clinical data analysis. Even a basic knowledge of the SAS can help you be more employable.
In CDM, SAS is used for the following purposes:
- Running validation checks
- Creating reports
- Cleaning datasets
- Generating listings
- Automating quality checks
Note: Learning SAS is one of the most smartest ways to make a long term career in Clinical data Management
A Step By Step Road Map for Pharmacy Students to enter CDM
Here is the roadmap that will help you get started and successful in your CDM career.
Step:1 Build your Clinical Research Foundation
Learn The basics of the following:
- Clinical Trial Phases
- Protocols
- ICH-GCP
- Roles of CROs
- Data Collection Methods
Try learning online beginner courses which is enough as a fresher.
Step 2: Learn Core CDM concepts
Focus mainly on:
- CRF design
- CDM lifecycle
- Query Management
- Data Management
- Data validation
- Data cleaning
- UAT( User Acceptance Testing)
- Database Lock
All of these can be your foundation for your CDM jobs.
Step 3: Get Hands-on Training In CDM Tools
Basic Familiarity with the tools like Rave, Oracle clinical, Or Veeva Vault CDM will set you apart from the others.
Note: Many training institutes like Biotecnika offer internship programs that also offer tool-based learning.
Step 4: Learn SAS
SAS is not mandatory for a fresher but it helps you with the improvement in your career growth.
Learn Basics like:
- Data Steps
- PROC SQL
- PROC SORT, PROC PRINT
- Data cleaning commands
Step 5: Prepare an industry-focused Resume
Highlight the following while preparing your resume
- Pharma Education
- Clinical research knowledge
- CDM skills
- Tools& SAS Exposure
- Projects and internships
- Certifications
Step 6: Apply For Positions Made For Freshers
Search for the following roles:
- CDM Associate
- Clinical Data Coordinator
- Data Validation Trainee
- Medical Coder
- Data Management intern
Many CROs like IQVIA, ICON, and Paraxel are regularly hiring pharmacy graduates.
Expected Salary In Clinical Data Management (India)
The salaries vary based on the company and skills you develop.
- Entry level (0-1 year)
₹3,00,000 – ₹5,00,000 Per Year
- Mid Level (2-4 Years)
₹6,00,000 – ₹12,00,000 Per Year
- Senior Level (5+Years)
₹13,00,000 – ₹20,00,000 Per Year
- With SAS Specialization
Salary jumps to 20-40% more.
Common Interview Questions For CDM Jobs After B Pharma
Here are the most commonly asked questions that you should also prepare
- What is Clinical Data Management?
- What is CRF?
- What is a Query in CDM?
- What is SAE Reconciliation?
- What are CDM Tools you know?
- What is SDTM? (if you know SAS)
- Why do you want to join CDM ?
- What are the Phases of Clinical Trials?
If you’re confident enough to answer these questions and cover these topics then you can crack fresher level interviews.
Conclusion
Starting a Clinical Data Management career after pharmacy graduation is one of the smartest choices you can make in today’s growing clinical research industry. With the right skills and tools, clinical trial knowledge, medical coding, data validation and basic SAS, you can quickly enter the field and grow into a high paying job role.




