High-Paying Non-R&D Pharma Jobs in India (2026 Career Guide)
When Riya finished her M.Pharm, she imagined herself to work in a white lab coat, with glass beakers, and long hours in a research lab. That’s what everyone like her thought “pharmacy jobs” meant.
But six months into job hunting, she realized something surprising, the best-paying pharmaceutical jobs were not only in R&D, there are so many Non-R&D Pharma Jobs as well
Her seniors were working as regulatory specialists, pharmacovigilance officers, data analysts, and clinical operations associates. They were earning more, working with global clients, and some were even working from home at their comfort.
The moment she understood this, her entire perspective on her career path changed.
Fast forward to today, Riya manages global submissions for a top pharmaceutical company and earns more than she ever expected. And all this happened without her stepping into a research lab.
And she’s not alone. Thousands of science and pharmacy graduates across India are finding success in non-R&D pharma roles. These are the pharmaceutical industry careers that blend science, business, data, and technology.
In this career guide, we will look into the top non-R&D pharma jobs in India, that pays more than you have ever thought, the skills you need, and how you can start your career, even if you’re just graduating or looking to switch your career.
List of High-Paying Non-R&D Pharma Jobs in India
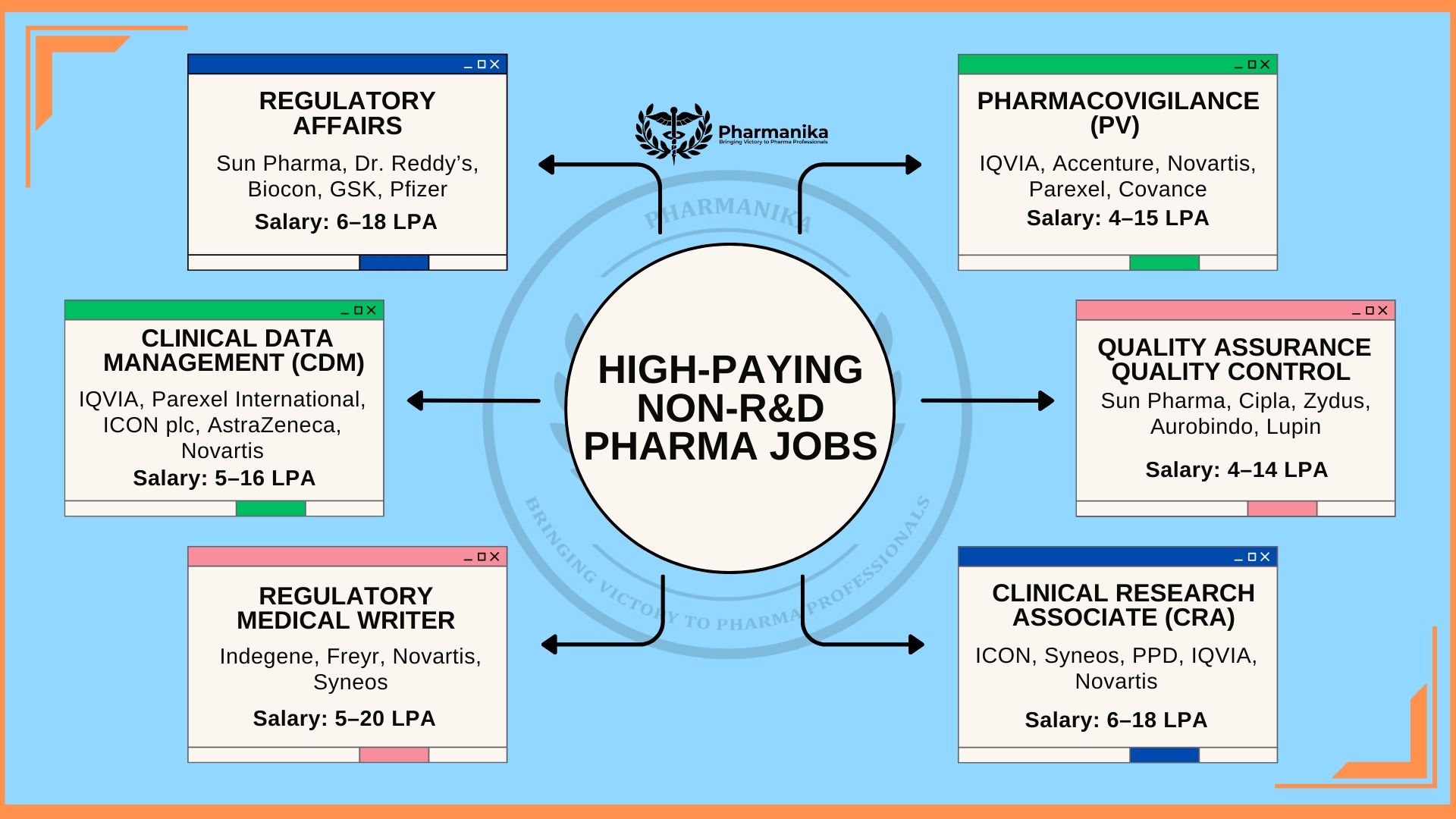
Regulatory Affairs Specialist
- Role of RA Specialist: Regulatory Affairs professionals are the people who are aware of all the guidelines and standards. They make sure that products meet all of them before they enter into the market. They also prepare documents for CDSCO, FDA, EMA, or WHO approvals.
- Average salary: 6–18 LPA (can go upto 20+ LPA in global companies for experienced professionals).
- Recruiters who actively hire: There are a few companies like Sun Pharma, Dr. Reddy’s, Biocon, GSK, Pfizer who actively hire candidates. These companies also give equal opportunity for freshers.
- Skills required to secure a job: Apart from theoretical knowledge that you learn in college, there are a few skills you need to master. They are regulatory guidelines, documentation, global submission formats, and strong communication.
Tip: There are a few certification courses in Regulatory Affairs that will help you enter into the field faster. For example RA program offered by Biotecnika can make you set a strong foundation.
Pharmacovigilance (Drug Safety Officer)
- Role of a drug safety officer: Monitor, record, and evaluate adverse drug reactions to ensure patient safety post-launch.
- Approximate Salary : The expected salary for the roles in pharmacovigilance range ₹4–15 LPA
- Top companies that hire for these roles: IQVIA, Accenture, Novartis, Parexel, Covance are a few companies who work in pharmacovigilance and offer opportunities for young graduates.
- Required Skills: Linst of skills required for this field include MedDRA, case processing, signal detection, medical coding.
This field has a high pay because every drug that is sold in the market across the world needs safety monitoring. As there is a rising demand for this, there are many pharmacovigilance hubs set up in India.
Clinical Data Management (CDM) Professional
- Role of people working in CDM: These experts collect and manage clinical trial data. They also ensure that it’s accurate, complete, and follow all the regulatory guidelines
- Average salary: ₹5–16 LPA.
- Important Skills: GCP guidelines, EDC tools (Oracle, Medidata), attention to detail.
- Career path: The career path from a fresher to an experienced professional takes a few years and the flow goes like this. Data Coordinator → Data Manager → Project Lead.
Career tip: If you want a strong career in this field, combine CDM with data analytics skills. This will help you transition to global data science roles.
Quality Assurance (QA) and Quality Control (QC)
Role of QA/QC Experts: Professionals working in this field are involved in the maintenance and product quality and compliance for drug manufacturing jobs.. They do it through documentation, audits, and regular lab checks.
Approximate Salary: The expected salary range is around ₹4–14 LPA. The range varies depending on experience and company.
Required Skills: Here are a few important skills : GMP, SOP preparation, analytical techniques, problem-solving.
This career is hight rewarding because of various reasons: QA/QC are the backbone of manufacturing and these professionals ensure safety and consistency of every batch produced. This job also requires a lot of precision and which is why it is highly rewarding.
Regulatory Medical Writer
Job Role: Medical writers are involved in the preparation of regulatory and clinical documents such as trial summaries, investigator brochures, and submission dossiers.
Salary Range: The expected salary range is ₹5–20 LPA. In this field freelancers can earn more.
Skills: Major skills required for a successful career are Scientific writing, English proficiency, understanding of ICH-GCP.
Additional tip: This career is ideal for students who love science and writing. This also gives professional opportunity to work remotely and do freelancing.
Clinical Research Associate (CRA)
Work of CRA Professionals: The CRA professionals perform various tasks everyday. The different tasks include: Clinical trials monitoring, ensuring protocol adherence, and maintain the quality of data collection done at sites.
Salary Range: Salary for CRA professionals range upto ₹6–18 LPA.
Companies that recruit candidates: Some of the famous companies that give opportunity for candidates are : ICON, Syneos, PPD, IQVIA, Novartis.
Important Skills: Some of the important skills required to work in this field are GCP, communication, site monitoring, reporting.
Career Tip: This job role is the best for those candidates who are interested in clinical operations without involving direct lab work.
Important Skills that will get you Non R&D Pharma Jobs
Every non-R&D jobs require a set of specialized knowledge and soft skills. Here’s what recruiters look in a candidate:
Technical Skills
- GxP and GMP standards
- ICH-GCP guidelines
- Regulatory and clinical documentation
- Data analytics tools (Excel, Power BI, SQL)
- EDC systems and pharmacovigilance databases
Soft Skills
- Communication and presentation
- Attention to detail
- Global regulatory understanding
- Teamwork and adaptability
- Problem-solving mindset
In today’s job market there is a heavy competition amongst the candidates. If you have to stand unique from the talented crowd there are additional certifications and courses that will boost your chances of getting a job and pay. The list of courses are:
- Clinical Data Management
- Pharmacovigilance and Drug Safety
- Regulatory Affairs (RAPS or Biotecnika)
- Pharma Business Analytics
- Quality Management
Getting experience by doing these courses are important, but what is more important is that doing these courses from the right place. The certificate you earn should be authorized and add value to the time and efforts you put. Do thorough research and ensure you choose the right course. Here are list of courses offered by Biotecnika and you can refer to them for an idea.
How to Enter These Roles
It is a myth that only experienced professionals have a place in this field. But the truth is that you don’t need years of experience to start. With the right skill set and preparation, you can enter most of these roles immediately after you graduates as a fresher.
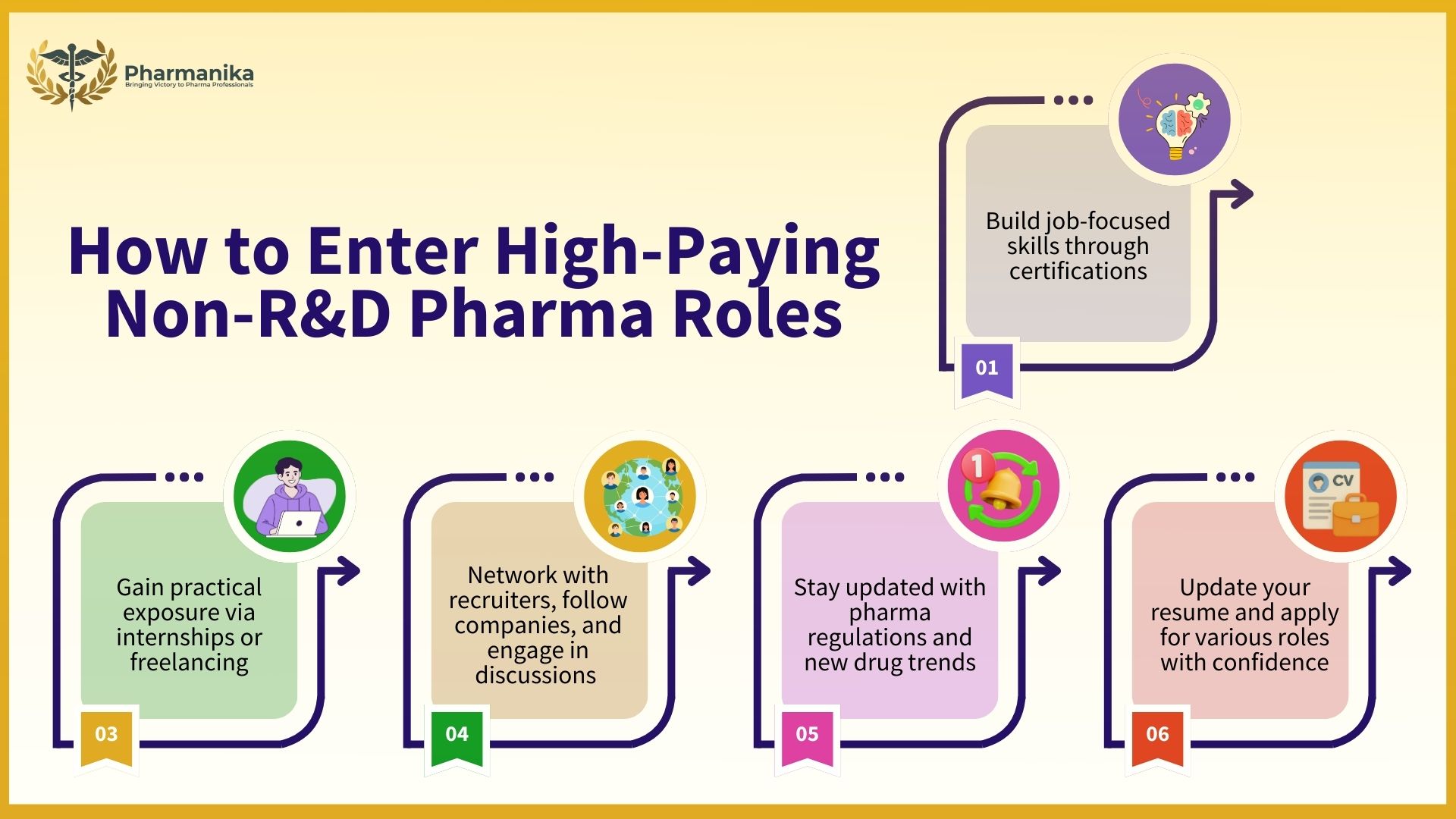
Here’s how you can work towards it:
- Take short-term certification courses to build specific skills required for pharmacy jobs
- Do internships with CROs or pharma startups. Network with people and get contacts to find internship opportunities.
- Use LinkedIn to follow recruiters, connect with professionals, and engage in industry discussions. This will let you stay updated about your field.
- Start freelancing (medical writing, documentation) to gain practical exposure. Freelancing gives you an opportunity to work with different kings of people and will prepare you for the industry.
- Stay updated with regulatory news, new drugs, and AI applications in pharma.
Future of Non-R&D Pharma Jobs
In this section, we will see what will the non-R&D pharma career look like from 2026-2030. The future for this field is bright and strong.
There are the few things that will shape the field in the next decade:
- AI and automation: This area is redefining data, compliance, and analytics roles.
- Remote and hybrid jobs are growing, especially in writing and data management. People love working from their comfort zone at their place.
- Global exposure: In recent times we can see that Indian professionals are hired more for projects abroad.
- Cross-disciplinary skills: Back were the days when people were working only in one field. Today cross disciplinary job roles are gaining more attention. For example, science + data + communication will lead to top-paying positions.
By 2030, the most successful pharma professionals will be those who understand science and also data, and regulation.
The Indian pharma industry is full of opportunities and you know what is the best part? You don’t need to be a researcher to make an impact or earn well.
From regulatory affairs to data analytics, from drug safety to product marketing, these careers merge science, business, and technology. These careers offer stability, growth, and gives a good pay among the highest paying pharmaceutical jobs in India.
So if you’re a student wondering what is there beyond a research lab, remember this:
Pharma in 2026 isn’t just about new discoveries, it’s about direction, compliance, and innovation.




