Scope of Pharmacy: How to Switch from Pharmacy to Regulatory Affairs or Clinical Research
Are you thinking of switching your career from pharmacy and want to know the Scope of Pharmacy and how to switch to regulatory affairs or clinical research? There was a girl named Riya who was very confined to traditional roles in a retail pharmacy. Today, she is a regulatory affairs associate in a leading pharmaceutical company. She plays a significant role in bringing out safe and effective medicines to the public. Like her, many graduates are switching their career paths from traditional to nontraditional, which will help them face dynamic challenges and achieve global impact.
If you are a Pharmacy Graduate and have completed your Pharmacy Degree, who feels as if you’re stuck between conventional roles or searching for a significant and futuristic career path, you’re not alone. The demand for skilled professionals in Regulatory Affairs(RA) and Clinical Research(CR) is increasing day by day.
This growth is mainly because of the pharmaceutical companies that are increasing attention on regulatory compliance, innovation, and patient safety. Both RA and CR make the individual more scientific, analytical, and expert in documentation, which every pharma student needs it make the transition smoother and more rewarding.
This guide will help you walk through everything you need to know to switch from pharmacy to regulatory affairs or clinical research. It covers the basic understanding of career landscapes, skill building, gaining relevant experience, and securing your freshers’ job roles. This guide will help you transform your pharmacy background into a rewarding career in higher-growth domains.
Introduction
Many Pharmacy Graduates are exploring these non-traditional pharmaceutical career paths, such as Regulatory Affairs(RA) and Clinical Research (CRA). These roles mainly focus on drug safety and efficacy through clinical studies. The pharmaceutical world is evolving beyond the traditional roles, which include the hospital pharmacy. Regulatory Affairs and Clinical Research is a high-growth career demand in the pharma world, which is relevant to pharma graduates to upskill. RA focuses on making sure that drug safety, laws, and regulations while CR focuses on testing the drug products to check for the safety and effectiveness of the drug, which will go through proper clinical trials.
You want to know what is new in Pharmacy As a career, so this guide helps you to walk you through the transition from pharmacy to these growth career fields. From understanding the career landscape and comparing between RA and CR paths for skills, certifications, and experience, this guide will help you for a rewarding career in Regulatory Affairs or Clinical Research.
Understanding the Career Landscape and Scope of Pharmacy
What is Regulatory Affairs?
Regulatory affairs professionals make sure that the pharmaceutical products comply with the regulatory laws and regulations from the making of the product that is from the development of the product to the marketing of the product. Their work also involves preparing and submitting the regulatory documents for the products, gaining approvals for the product, and maintaining compliance after launching the product. RA plays a significant role in product development, marketing, and pharmacovigilance, helping companies navigate complex regulatory environments globally.
What is Clinical Research?
Clinical Research Professionals’ main aim is to conduct designed trials on human participants to evaluate the safety, efficacy, and overall performance of the drugs and medical products. This process is conducted in different phases (I-IV), in each phase, there is an increasing number of participants, and objectives are changed, strictly adhering to the ethical guidelines and Good Clinical Practice (GCP) standards.
Why These Fields Attract Pharmacy Graduates
The pharmacy graduates have a strong knowledge of drug formulations and drug safety, which is more directly applicable to RA and CR. These roles offer high growth, international demand, and exposure to research and regulatory. These are the roles that are more futuristic and do not involve any sales, but indeed progress your career growth and global opportunities.
Comparing the Two Career Paths
| Aspect | Regulatory Affairs | Clinical Research |
| Nature of Work | Compliance, documentation, submissions | Clinical trials, data collection, and monitoring |
| Core Skills | Documentation, legal, and regulatory understanding | Data analysis, patient safety, and trial management |
| Ideal for | Detail-oriented, policy-driven individuals | Those interested in research and patient interaction |
| Career Progression | Regulatory Associate → Manager → Director | Clinical Research Associate (CRA) → Project Manager → Clinical Scientist |
| Salary Range (India) | ₹4–10 LPA | ₹3.5–9 LPA |
Pro Tip: If you are interested in working with paperwork, polices, and legal framework, then you can go with Regulatory Affairs as your growth career. If you are interested in research, clinical trials, and interactions with patients, then opt for Clinical Research.
Why a Pharmacy Degree is Significant?
For RA and CR roles, the pharma degree provides knowledge about pharmacology, toxicology, drug formulations, and therapeutics. The degree gives safety expertise, which is significant for clinical trials. Employers demand pharmacy graduates because they have basic regulatory understanding, drug safety, and effectiveness, which makes more easier to switch to RA or CR.
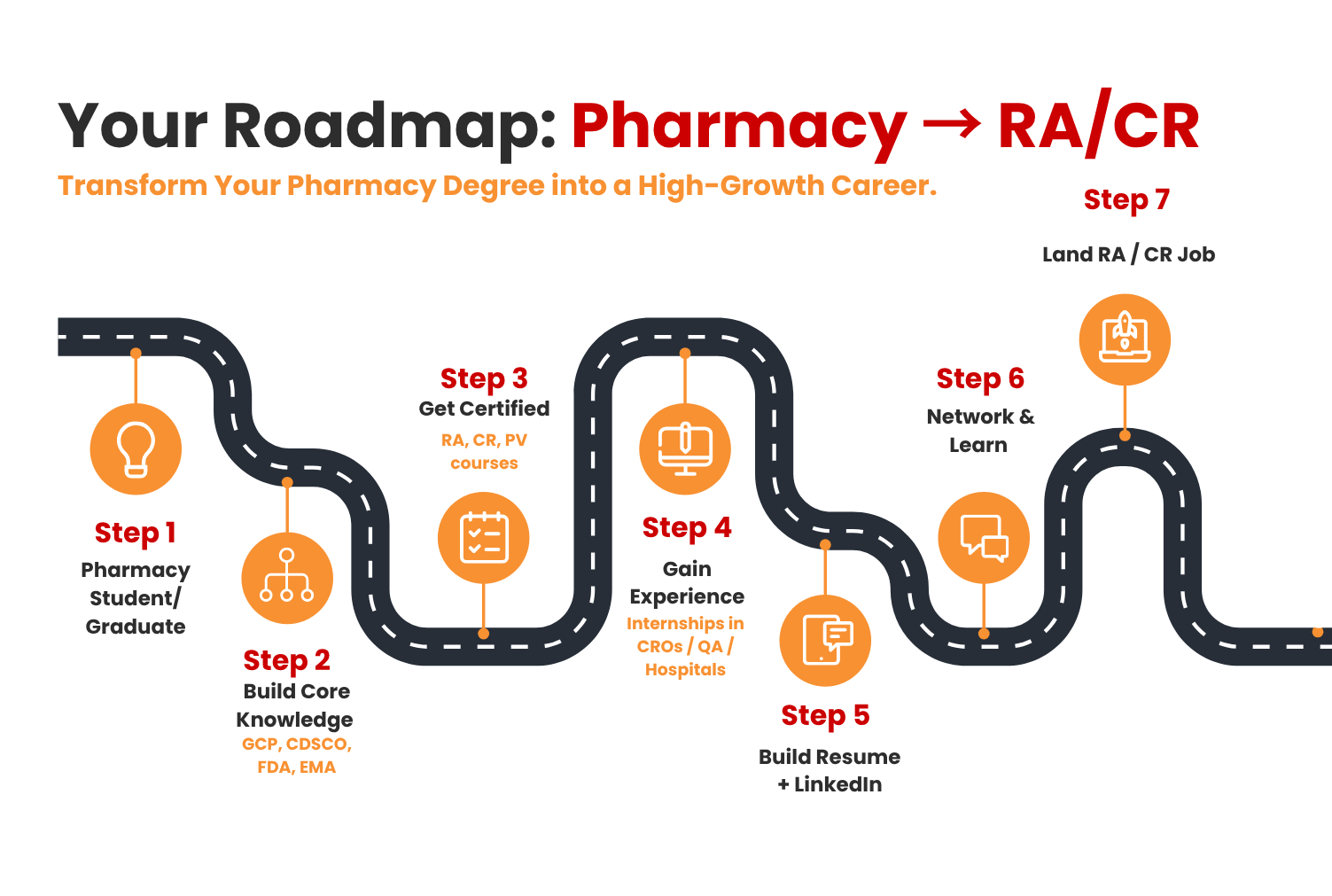
Detailed Guide for Career Transition and Scope of Pharmacy
- Self-Analysis and Clear Objective
Have a clear mindset. If you’re going to choose Research and patient engagement or documentation and regulatory compliance, you can use online career portals, which will help you decide.
- Build Core Knowledge
To build your knowledge, you should have knowledge of regulations and guidelines, which include: ICH-GCP for clinical research, CDSCO(India), FDA(US), Eand MA(Europe). These are the important regulations required for a career in regulatory affairs. Make use of free resources like WHO, NIH, and NPTEL to build a strong knowledge foundation.
- Get Certified or Trained
Apart from technical knowledge, certifications add a lot of value to your profile. You can get that by enrolling in targeted courses like Regulatory Affairs Certification Programs, Clinical Research, and Pharmacovigilance Training. One can get these certifications from reputed portals like BioTecNika. Enhance yourself with project-based training and practical skills.
- Get an internship or work on Projects
You can start your internships from Contract Research Organizations(CRO’s), pharmaceutical quality assurance departments, and even in hospital research units. You know what helps you more is the hands-on internships, which add more value to your career, and also participate as a volunteer in clinical trials, which upskill your career.
- Make a Professional Resume and LinkedIn Profile
In your resume, add relevant skills that include skills based on clinical trials and documentation, which attract the readers and recruiters. Highlight only your main skills, which include drug safety and drug research. Also, in your resume, add a Trainee or Intern, which will attract the recruiters.
- Connections and Practical Experience
To enhance your connections and practical experience, consider enrolling in and joining professional associations such as the DIA (Drug Information Association), ISCR (Indian Society for Clinical Research), and RAPS (Regulatory Affairs Professionals Society). Additionally, consider attending webinars, conferences, and alumni events, as these can help you secure referrals. Also, keep an eye on experts on LinkedIn, which will help you stay updated.
- Apply for Beginner Roles
Don’t directly apply for Manager or scientist roles, but instead search and apply for beginner roles such as Regulatory Affairs Associate or Clinical Research Coordinator. Use job portals like LinkedIn, and also keep an eye on company career pages.
- Upskill and Stay Updated. On our daily basis, we know everything is changing every day, so we have to keep upskilling ourselves according to it. Upskill yourself with short courses and clinical trials, which will be useful for your career.
Job Roles / Career Progression and Scope of Pharmacy
- Regulatory Affairs Career Pathway
To start your career in Regulatory affairs, first join as a Trainee or as an intern, which will help you progress to future roles such as a manager and a scientist. Opportunities do exist; everything comes from us. There are government agencies such as the FDA or MHRA.
- Clinical Research Career Pathway
To start your career and want to know the Scope of Pharmacy in Clinical Research, first join as an associate or as a coordinator, which will further help you to achieve your future positions. There are options such as hospitals and academia.
- Growth Expectation
The fields RA and CR are both categorized in growth careers, and they are the future careers that will create global demand, and also the main focus is where they will provide us with remote opportunities.
Required Skills and Qualifications
- Common Skills
The common skills include Strong communication, expertise in documentation, analytical thinking, and a basic understanding of drug development and safety.
- Regulatory Affairs Skills
Expert in submission portals like CTD and eCTD, basic knowledge of labeling, proper dose preparation, product registration, and regulatory guideline interpretation.
- Clinical Research Skills
Expert in data collection and analysis, adherence to GCP, trial management, monitoring patient safety, adverse event reporting, and use of clinical databases like EDC and Medidata.
Courses, Certifications, and Recommended Institutes
- Top Regulatory Affairs Courses: Postgraduate Diploma, RAPS Regulatory Affairs Certification (US).
- Top Clinical Research Certifications: ICH-GCP Certifications (NIH, WHO), PG Diploma in Clinical Research (ICRI).
- Online platforms like BioTecNika. Focus more on project-based and mentor-led learning for the best results.
Common Challenges
Pharmacy graduates mostly struggle with a lack of practical industry exposure, networking, and identifying proper entry roles. From QA/QC to RA/CR, suitable entry roles. Adapting from traditional roles like QA/QC to RA/CR requires mindset shifts and targeted skill development. Staying motivated during the early job search is crucial.
Real-Life Success Stories
Many pharmacy graduates have successfully transitioned by combining specialized training, internships, and active networking. For example, one graduate started with a QA role, gained a RAC certification, interned at a CRO, and secured a regulatory affairs associate role in a top pharma company.
Conclusion
Pharmacy graduates already hold a significant advantage with their scientific and regulatory foundation. By gaining focused training, practical experience, and networking strategically, they can unlock rewarding global careers in Regulatory Affairs or Clinical Research. Explore both paths carefully before committing to find your best fit.
Call to Action
Ready to know the Scope of Pharmacy and want to switch from pharmacy to Regulatory Affairs or Clinical Research? Start today by enrolling in a certified training program or internship. Follow professional pages and join industry associations to stay updated on opportunities. Transform your classroom knowledge into a global career by taking this important step now!




