Pharmacovigilance: Definition, Meaning & Role in Drug Safety
You might have heard of research, drug discovery, and clinical trials, as they are talked about more. But do you know what happens after these stages and once the drug has reached the market?
That is when Pharmacovigilance comes into the picture. The step that ensures every drug stays safe for millions of patients around the world.
We are in 2025, and pharmacovigilance is not a process that does the behind-the-scenes work. It is one of the fastest growing career paths in the life science industry. There is a new drug coming to the market every day and hence the demand for professionals who can analyze, monitor and report the drug safety has also increased.
Whether you’re a pharmacy, biotechnology, or chemistry graduate, understanding Pharmacovigilance will pave the way for a high-paying global career. Let’s explore what it is, why it matters, and how it can shape your future in the pharmaceutical industry
What is Pharmacovigilance?
Imagine a situation where you’ve just bought a brand-new smartphone. It’s sleek, fast, and loaded with new features. But soon after launch, users start reporting unexpected heating issues, glitches, or battery problems. The company immediately investigates, collects reports, finds the root cause, and releases a safety update.
This is the exact thing that happens with a newly approved medicine that enters the market. The entire process of tracking, investigating, and ensuring the safety of the product for patients across the world is what Pharmacovigilance does for drugs.
A drug comes to the market after years of clinical trials and validation. But when it is consumed by patients, there is a possibility for it to react differently due to various reasons. That is why monitoring does not stop at approval.
In simple terms, Pharmacovigilance (PV) is the system that involves detection, assessment, understanding, and prevention of adverse effects or other problems of the drug. Pharmacovigilance experts are the guardians of medicine.
Pharmacovigilance Definition
Pharmacovigilance (PV) definition is the science and activities related to detecting, assessing, understanding, and preventing adverse effects or any other drug-related problems. It is a critical part of the drug life cycle, bridging the gap between laboratory research and real-world patient care.
Key points to remember:
- Focuses on Adverse Drug Reactions (ADRs).
- Involves data collection, analysis, and reporting.
- Ensures regulatory compliance (FDA, WHO, EMA, or CDSCO) and public health protection.
History and Evolution of Pharmacovigilance
Pharmacovigilance was not used directly overnight. As the medicines and their complexity increased, the need for PV also became high. During the early 19th and 20th centuries, pharmacovigilance began as an informal observation of the side effects of drugs. The thalidomide tragedy in the 1960s, which caused thousands of birth defects. This let to the increase in the need for systematic drug safety monitoring. After this, regulatory bodies like FDA and EMA were created.
Today, pharmacovigilance is very proactive, uses AI, databases to make sure the medicines are safe throughout their lifecycle. The simple observation turned into advanced monitoring, making PV a very important part of drug development.
Key Objectives
The key objectives of PV are:
- To Ensure Patient Safety: Detect, assess, and prevent adverse drug reactions (ADRs) to protect patients.
- Monitor Drug Safety: Continuously track medicines post-marketing for unexpected side effects.
- To Reduce Risk: Identify potential risks and implement measures to reduce harm.
- Regulatory Compliance: Ensure all safety reporting meets national and international regulations.
- Improve Drug Use: Provide healthcare professionals with information to optimize safe and effective use
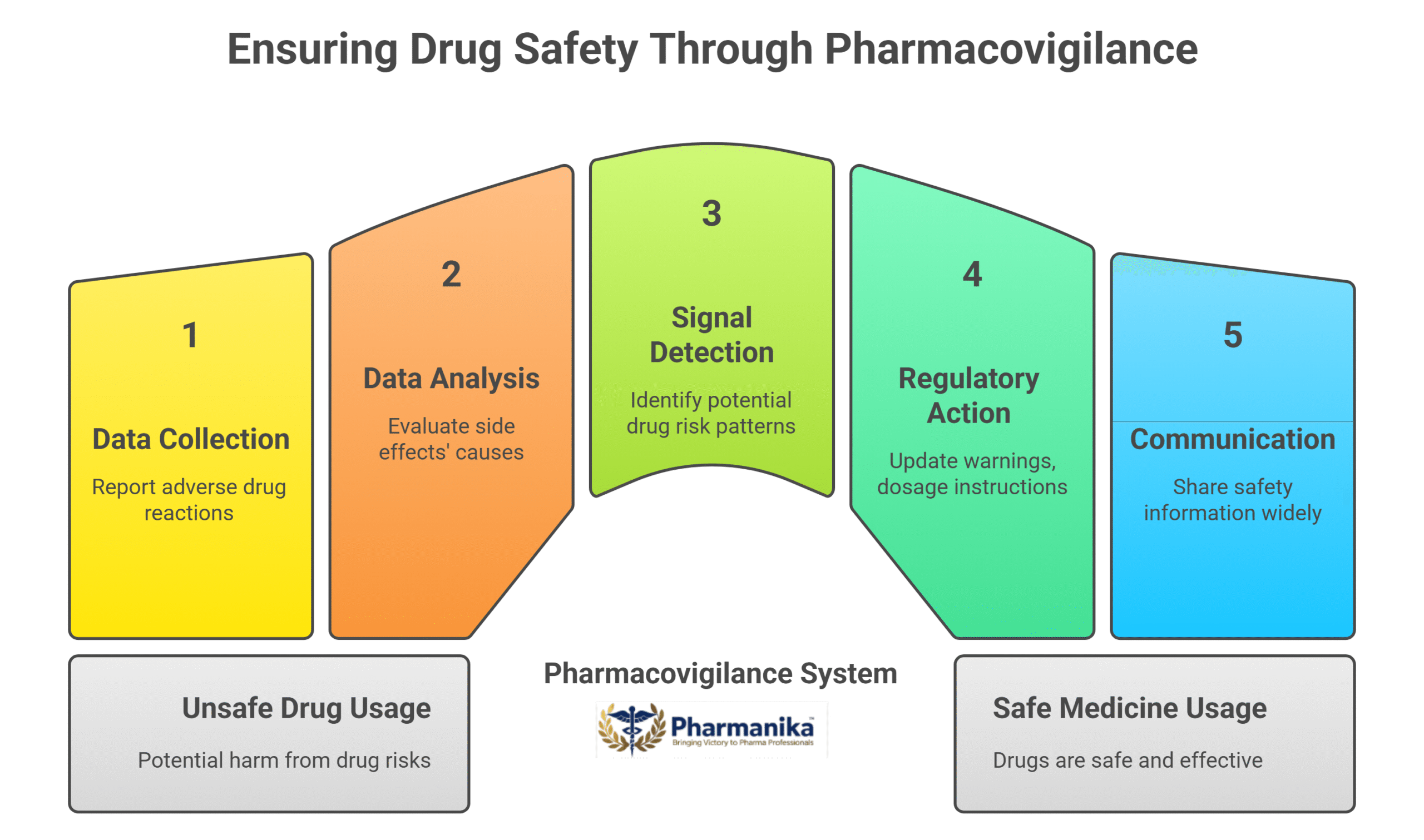
Pharmacovigilance works as a safety net for drugs. It plays a crucial role in working as a drug safety monitoring system. This ensures that the medicines are safe and are effective for the patients. It works as a process and the steps of the process are:
- Collection of Data: Adverse drug reactions (ADRs) are reported by healthcare professionals, hospitals, clinical trials, and patients.
- Data Analysis: Safety Experts analyze and evaluate if the side effects are linked to the drug or other causes.
- Signal Detection: To avoid any harm potential drug risk patterns are identified.
- Regulatory Action: Authorities like the FDA, EMA, and CDSCO update warnings, dosage instructions, or recall unsafe drugs.
- Communication: After the safety information is updates, it is shared with the doctors, pharmacists, and patients to promote safe use of medicine.
In short, pharmacovigilance is the backbone of drug safety in 2025, transforming raw reports into protective measures that safeguard public health.
Importance of Pharmacovigilance in Drug Safety
Pharmacovigilance is the first line of defense in ensuring drug safety. Even after going through clinical trials for so many years, some adverse effects might only appear after a medicine reaches a large group of people. By monitoring, detecting, and analyzing adverse drug reactions (ADRs), pharmacovigilance helps prevent serious health risks and protects patients worldwide.
Key benefits of include:
- Protecting Patients: Reduces the risk of harmful side effects and ensures medicines are safe to consume.
- Regulatory Compliance: Keeps pharmaceutical companies aligned with agencies like FDA, EMA, and CDSCO.
- Improving Treatment Outcomes: This step helps doctors make informed decisions for safe and effective use of the medicines
- Supporting Drug Innovation: Ensures new drugs enter the market safely, building trust among patients and healthcare professionals.
Overall, Pharmacovigilance plays a very important role and thanks to them for helping us consume medicines without second thoughts.
Pharmacovigilance Jobs
Pharmacovigilance is not just important for drug safety, it’s also a career path that is rapidly evolving in pharma and biotech industries. Recently the focus on adverse drug reaction monitoring, patient safety and regulatory compliance are more on focus, the companies are hiring actively for professionals. This will be the right chance for you to get a high-paying pharmacovigilance job.
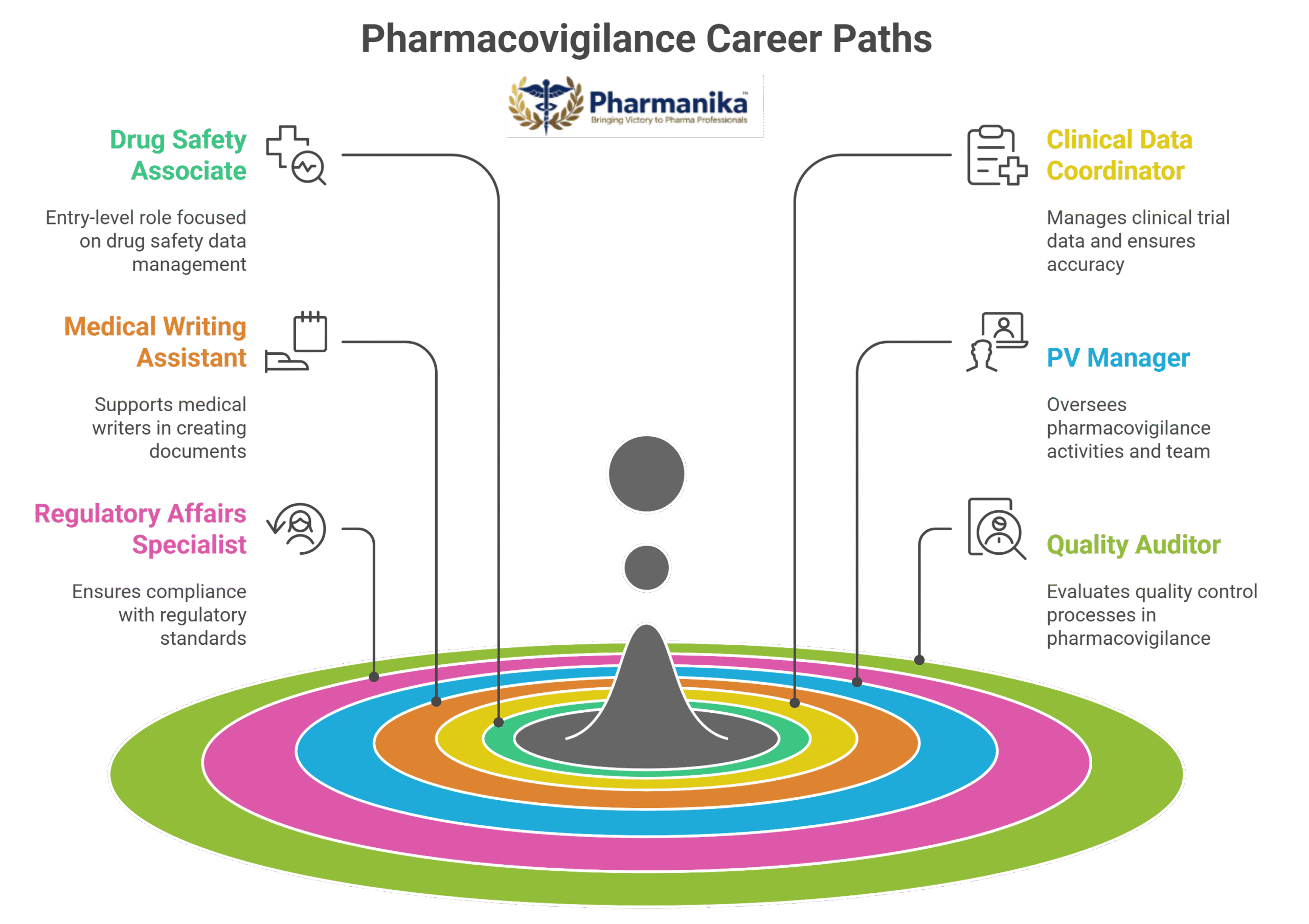
Pharmacovigilance Jobs for Freshers:
- Drug Safety Associate / PV Associate: This is an entry-level role handling ADR reports, case processing, and database management.
- Clinical Data Coordinator Role: These people assist in collecting and organizing safety data from clinical trials.
- Medical Writing Assistant: They support preparation of safety reports, regulatory submissions, and patient information.
Advanced Roles for Experienced Professionals:
- PV Manager / Safety Officer
- Regulatory Affairs Specialist
- Pharmacovigilance Quality Auditor
Why Choose a Career in Pharmacovigilance?
- This field has a rapidly growing job market in 2025.
- It gives you opportunities in top pharmaceutical companies, CROs, and biotech firms globally.
- This combines science, regulatory knowledge, and patient safety into a high paying career.
Pharmacovigilance jobs are ideal for freshers with a science and pharma background, analytical skills, and attention to detail, offering a strong foundation for a long-term career in drug safety.
Courses
As a fresher, to get a good pharmacovigilance job, gaining experience through enrolling in a specialized course can increase your employability and expertise.
Here are a few top courses:
- Certificate in Pharmacovigilance
- Duration: Few hours to a few weeks
- Content: Basics of drug safety, adverse event reporting, MedDRA, PV audits, and global guidelines.
- Ideal for: Freshers looking to gain foundational knowledge in pharmacovigilance.
Pharmacovigilance Certification Program
- Duration: 4–12 weeks
- Content: In-depth modules on case studies, regulatory affairs, safety database handling, and ICSR reporting.
- Ideal for: Graduates seeking roles like Drug Safety Associate or PV Scientist.
- Content: Practical training in case processing, data entry, medical coding, and PV software usage.
- Ideal for: Freshers who want industry-ready skills in drug safety.
There is a wide range of online and offline courses to choose from, and free online resources such as YouTube can also be very useful.
What is the Future?
The future will be driven by data, focused on patients and and technologically advanced, focusing on improving healthcare outcomes while ensuring regulatory compliance. Here’s what we can expect:
AI Integrated system: Automation and AI will dominate pharmacovigilance workflows, from detecting adverse analysis.
Automation & Robotic Process Automation(RPA): Routine tasks like case entry, data cleaning, and report generation will be handled by RPA tools, allowing professionals to focus on complex analysis and decision-making.
Patient-Centric Approaches: There will be new tools and apps that will empower patients to report adverse events directly, enhancing engagement and providing richer safety data.
The future of pharmacovigilance is moving toward a smarter, faster, and more patient-focused system where technology and data analytics will play a central role. For professionals entering the field, having knowledge on AI, RWD, automation, and patient engagement will be an advantage to staying ahead in the field.
From monitoring adverse reactions to ensuring regulatory compliance, pharmacovigilance protects patients and supports innovation in the pharmaceutical industry. For freshers and experienced professionals alike offers a high-paying pharmacovigilance job, global careers that combine science, technology, and patient care.
As the industry embraces AI, real-world data, and patient-centric tools, the future of pharmacovigilance is smarter, faster, and more impactful than ever. If you’re a pharmacy, biotech, or chemistry graduate, now is the perfect time to step into this dynamic field, safeguard public health, and shape the next generation of medicines.
So, what are you waiting for? Step into the exciting world of Pharmacovigilance and start making a real impact today. Hope this article answered all your questions.
Hope this article has answered all your questions about Pharmacovigilance and inspired you to explore exciting career opportunities in this growing field.






