Top Regulatory Affairs Career Paths in India & Abroad: Job Opportunities, Skills & Growth for Pharmacy Graduates
We know how the tablets are manufactured, but we should also know that every tablet, vaccine, and medical device that reaches millions of patients safely passes through the hands of Regulatory Affairs (RA) professionals. The field of Regulatory Affairs offers a rewarding career for those interested in ensuring compliance. These are the people who ensure that the drug meets all regulatory, national and international standards, maintain high quality and follow the rules of agencies like the CDSCO, FDA, EMA, and WHO.
In recent years, regulatory affairs jobs for pharmacy graduates have grown rapidly as India’s pharmaceutical exports and global collaborations are expanding. If you are a recent graduates or a professional dreaming of working in a multinational company, a clinical research organization, or a global regulatory agency, a career in Regulatory Affairs (RA) offers endless opportunities both in India and abroad.
In this article we will further discuss about the different career opportunities, employers, salary and tips to get a high paying job etc.
What Is Regulatory Affairs in Pharma?
Regulatory Affairs play an important role in the pharma, biotech, and medical device industries. It bridges the gap between companies that develop medical products and the government authorities that approve and monitor them.
Every medicine or device that is in the market is safe, effective and compliant with all guidelines because of Regulatory Affairs. They ensure all these before it reached patients.
Role of Regulatory professionals:
- They prepare and review dossiers (technical documents submitted to authorities)
- Product registrations in different countries are handled by them
- They ensure labeling and promotional compliance
- With regulations changing now and then, they keep the products updated
A career in RA requires patience, precision, and passion. It is a perfect blend of science and law for pharmacy and life science graduates.
For pharmacy graduates too know how compliance, documentation, and safety standards come together, they should have understanding of regulatory affairs roles and responsibilities.
Regulatory Affairs Career Path in India
India is one of the largest producers and exporters of medicines across the world. Because of that, the demand for skilled regulatory professionals has grown across various sectors like pharma, biotech, and medical device. With this, there are also increased jobs in Regulatory Affairs for Pharmacy Graduates
Entry-Level Roles
Here are a few job roles for freshers. If you have recently graduates then you can focus on applying for these roles.
- Regulatory Affairs Associate / Trainee: This role involves the preparation of technical documents, assisting with product registration, and coordinating with QA and R&D teams.
- Regulatory Documentation Executive: Manages labeling, packaging inserts, and product dossiers.
- Regulatory Assistant: Supports database maintenance and correspondence with authorities.
Tip: Fresh graduates can begin as interns or trainees and gradually move into full-time regulatory positions.
Salary Tip: The entry-level regulatory affairs associate India salary ranges from ₹3–5 LPA depending on company and project exposure.
Mid-Level Roles
- Regulatory Affairs Officer / Specialist: Leads submissions, reviews product variations, and communicates with agencies.
- Regulatory Affairs Manager: Supervises teams, manages multiple product portfolios, and ensures compliance with new regulations.
Senior-Level Roles
- Head of Regulatory Affairs / Director of Compliance: Designs global submission strategies, manages audits, and leads interactions with international agencies.
Companies that hire in India:
- Pharma Companies: Sun Pharma, Cipla, Dr. Reddy’s, Biocon
- CROs: IQVIA, Veeda, Syngene
- Medical Devices: Medtronic, Siemens Healthineers, Abbott India
- Consultancies: Parexel, Accenture, Deloitte Life Sciences
With the government’s “Make in India” and “Pharma Vision 2030” initiatives, there is an expectation of Regulatory Affairs roles in India to increase, which will create more opportunities for skilled professionals.
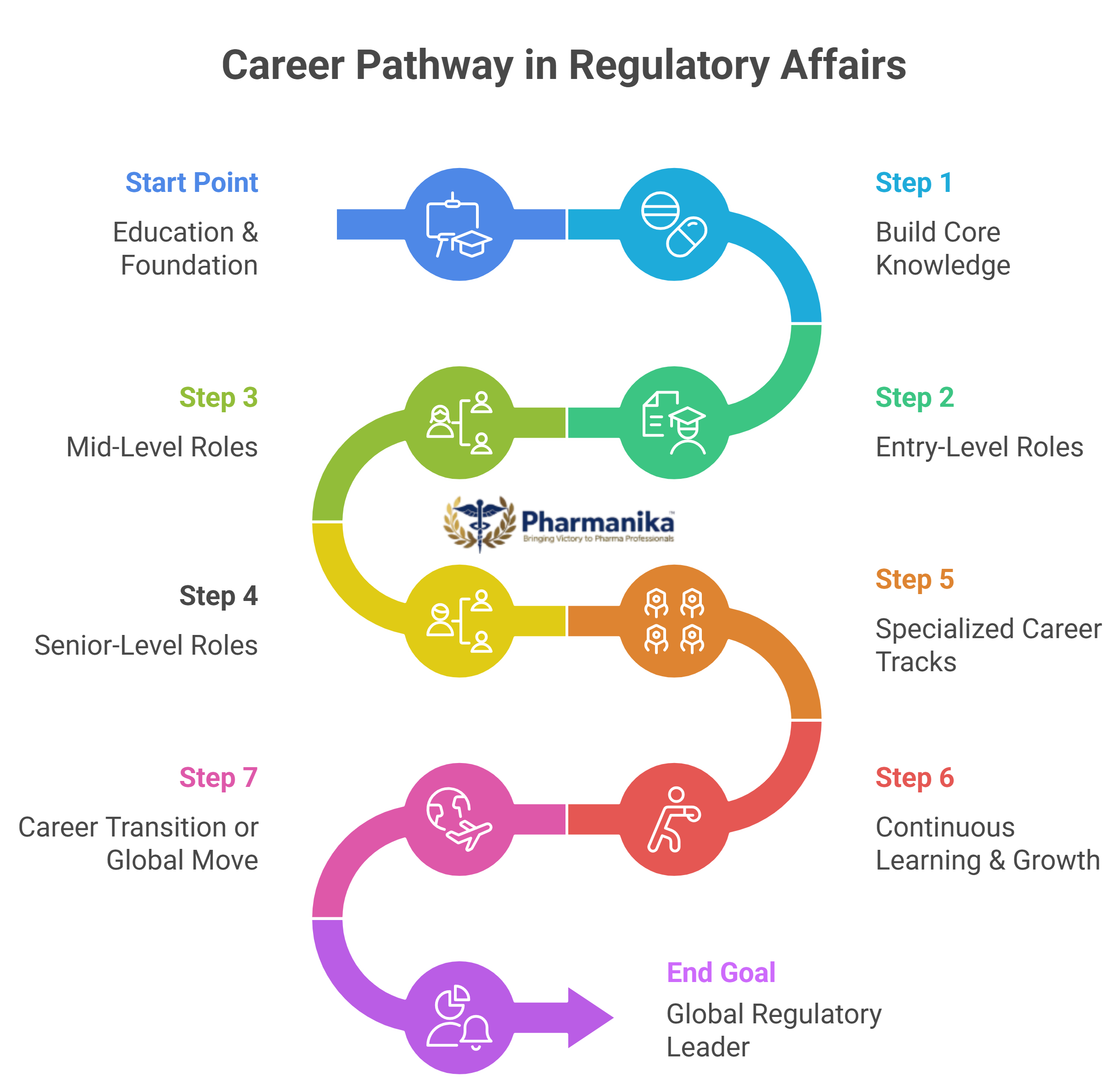
Regulatory Affairs Career Abroad
There is a lot more scope for regulatory affairs abroad. They have clear hierarchies with specialized roles. Professionals with international certifications and experience in global guidelines (FDA, EMA, MHRA, TGA, Health Canada) are in high demand for the industry. For pharma graduates to build a career in regulatory affairs abroad, one has to be familiar with global agencies like the FDA, EMA, and MHRA. Here are the career opportunities in different countries:
United States
- You can work with the FDA (Food and Drug Administration) or leading pharma companies like Pfizer, Merck, or Johnson & Johnson.
- The different roles in which you can work are Regulatory Specialist, Labeling Manager, or Submission Coordinator.
Europe (UK & EU)
- The EMA (European Medicines Agency) and MHRA (UK) focus on centralized approvals across EU countries.
- In Europe, RA experts with ICH and EU guideline knowledge are highly valued and are in demand.
Canada, Australia, and Singapore
- These places are known for strong pharma and biotech sectors.
- Regulatory professionals often handle product registration and post-market surveillance for the Asia-Pacific regions.
Fact: Many Indian pharmacy graduates build global careers by starting in Indian RA departments handling international submissions, then moving to regional roles abroad.
Essential Skills for a Regulatory Affairs Career
Technical skills are important, but they are not enough to succeed in regulatory affairs career. One should also master communication, compliance, and coordination.
Important Skills
Here are a few major skills that are important for regulatory affairs career.
- Regulatory Knowledge: One has to have an understanding of national and global regulations (CDSCO, FDA, EMA, ICH).
- Documentation Expertise: This expertise includes dossier preparation (CTD/eCTD formats), labeling, and SOP writing.
- Attention to Detail: Product approvals get delayed by even a small mistake in documentation. So one has to pay attention to the smallest details.
- Communication Skills: When working in a regulatory field, there will be regular interactions with QA, R&D, manufacturing teams, and government bodies. Hence having good communication skills is important.
- Project Management: When there is a lot of work, one should handle multiple submissions in short time. This required good time and project management skills.
- Technical Skills: Familiarity with electronic submission systems and database management are important
If you’re looking to strengthen these core skills and build a strong foundation for your pharma regulatory career, Biotecnika’s Regulatory Affairs Certification Course is an excellent starting point.
The program is designed for pharmacy, biotech, and life science graduates who want hands-on exposure to real-world regulatory processes.
LINK FOR THE REGULATORY AFFAIRS COURSE
Salary Range in Regulatory Affairs
| Career Level | India (Annual) | Abroad (Approx. Annual) |
| Entry-Level (Associate) | ₹3 – 5 LPA | $45,000 – $60,000 |
| Mid-Level (Manager) | ₹6 – 10 LPA | $70,000 – $90,000 |
| Senior (Director/Head) | ₹12 – 25 LPA | $100,000+ |
Note: Salaries depend on product type (drug, device, biologic), company size, and experience. Global experience or RAC certification can significantly increase earning potential. These are just approximations and can vary with respect to time and other factors.
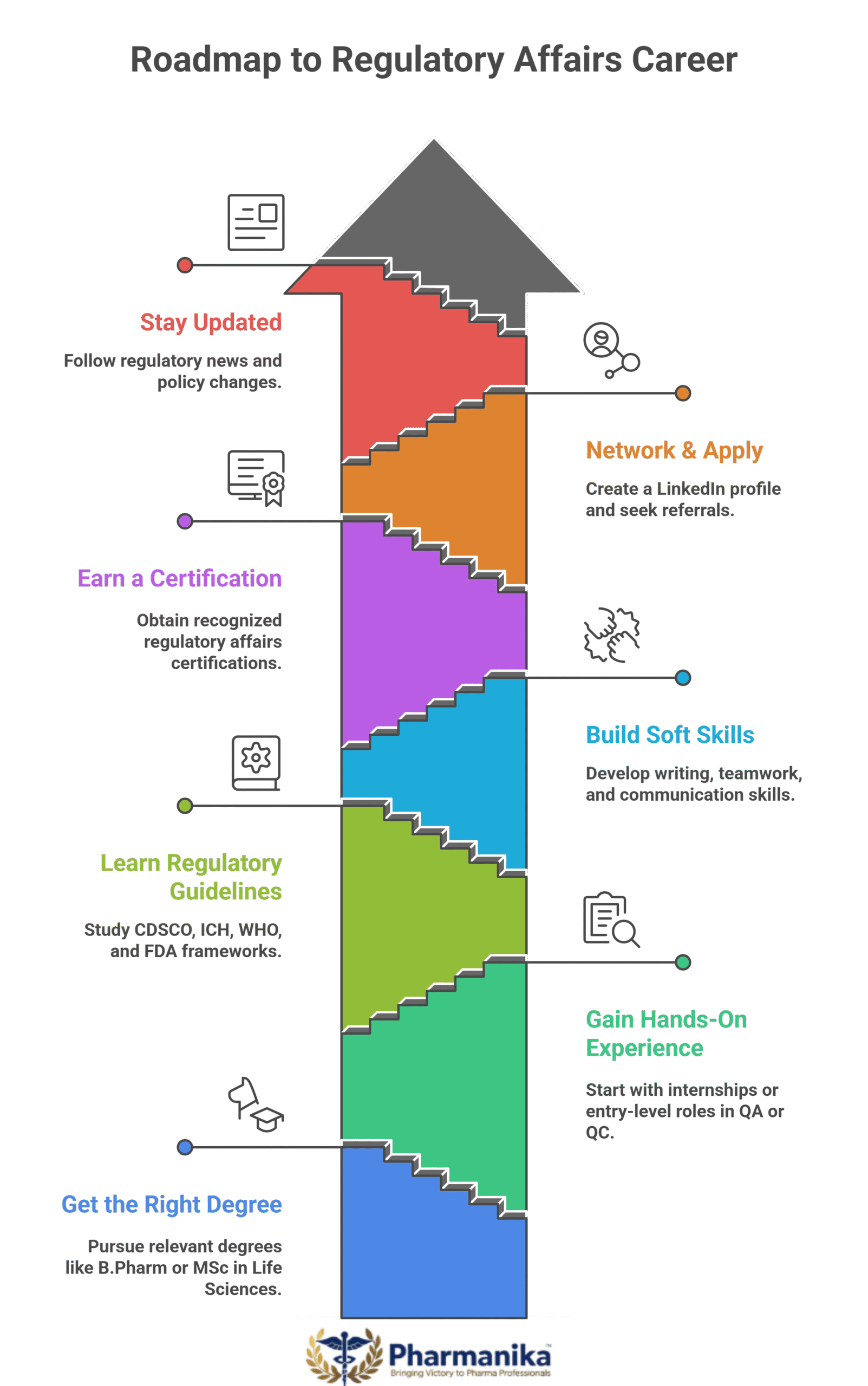
Roadmap to start your career
If you’re a pharmacy graduate or a life science student aiming to enter this field, here’s a simple roadmap:
- Get the Right Degree: Your career base starts with the right degree. Focus on pursuing degrees like B.Pharm, M.Pharm (Regulatory Affairs), MSc (Life Sciences/Biotech).
- Gain Hands-On Experience: Along with the degree getting trained hands-on is important. Start with internships or entry-level roles in QA, QC, or documentation.
- Learn the Basics of Regulatory Guidelines: Regulatory guidelines are the core for this career. Leaver various guidelines like CDSCO, ICH, WHO, FDA frameworks.
- Build Soft Skills: Technical knowledge combined with good soft skills gives you a hight paying career. Focus on skills like writing, teamwork, time management, and regulatory communication.
- Earn a Certification: To have an additional advantage and to stand out in this competitive field, take certification courses. Choose a recognized regulatory affairs course or diploma.
- Network & Apply Strategically: Create a LinkedIn profile highlighting RA skills, certifications, and any dossier-related experience. Network with the right people and get referrals.
- Stay Updated: Follow regulatory agencies and pharma news portals to know about new drug approvals and policy changes. To stay ahead in the field, you should be updated with latest information.
Career Tip: Many professionals transition from Quality Assurance or Clinical Research roles into Regulatory Affairs after gaining 1–2 years of industry experience.
Regulatory Affairs is more than a job, it’s an entry to understanding how science, business, and law intersect to protect health of the public. It is a career that promises constant learning, growth and global mobility especially for pharmacy graduates.
If you are someone who is looking to start your career in India or aim for regulatory affairs abroad, this profession offers a meaningful, long-term path in the pharmaceutical industry. With the right regulatory affairs skills, certifications, and continuous learning, you can be part of the policies that shape the future of healthcare.
Remember that every approved drug tells a story, and regulatory professionals are the authors behind it.




